RNA interference or RNA silencing is the process in which the translation of a protein is suppressed for a certain time by injecting the corresponding double-stranded RNA. This targeted inactivation of genes is often used in the study of cellular processes in vivo .
Biological background
The phenomenon of RNA interference was first detected in the C. elegans roundworm , but later also in other eukaryotes. It is a natural defense mechanism against pathogens. The mechanism is based on the detection of certain conserved pathogen markers, in this case double-stranded RNA. Double-stranded RNA occurs as an intermediate in the replication of many viruses, but cannot be produced by the infected cells themselves. In response to the presence of this RNA, the process of RNA interference is activated.
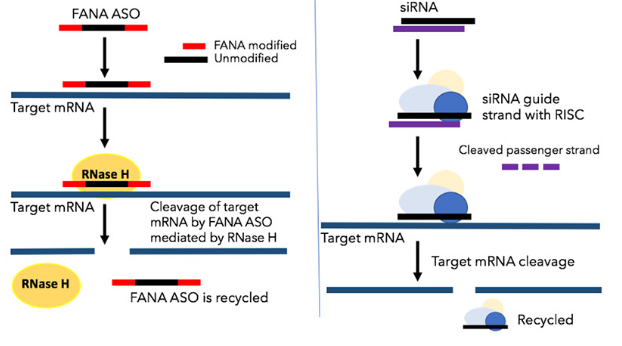
The RNase Dicer enzyme cuts the double-stranded RNA into small fragments that span 21 to 23 base pairs. These fragments are called small interferring RNA (siRNA). They are then incorporated into the RNA-induced silencing complex (RISC). The RISC unwinds the fragments in an ATP-dependent reaction, where upon the now single-stranded RNA molecules finally direct the RISC to complementary mRNA molecules of the pathogen and mediate the degradation. RNA interference occurs in plants, invertebrates and vertebrates.
Possibilities of sequence-specific knockdown
It has long been known that the injection of double-stranded RNA leads to sequence-specific degradation of the complementary cellular mRNA. Knowing that this phenomenon is also conserved in higher eukaryotes enables it to be widely used in life sciences. RNA interference can be used to sequence-specifically inactivate genes. At least theoretically, the production of each protein can be specifically switched off using synthetic siRNAs. The short RNAs do not trigger an interferon response and are extremely sequence-specific. Accordingly, gene silencing takes place at the level of translation, not transcription. In any case, the inhibition is only transient.
The advantage of FANA oligos
The RNA interference pathway involves the participation of the RNA-induced silencing complex (RISC). SiRNAs are processed in the cytoplasm. In contrast, FANA single-strand antisense oligonucleotides use RNase H-mediated cleavage. This is simpler and eliminates RISC-associated off-target effects that are commonly seen with siRNA. FANA oligos enter the cell nucleus and are used for the targeted analysis of the RNA present in the cell nucleus. FANAs do not require transfection reagents or transmission reagents. They are manufactured ready for use by our partner AUM-Biotech.
Our partner AUM Biotech produces the FANA oligos specific to your research questions. RNA silencing products are available for bacteria, viruses and cellular models, but also for insects, fish and other animal studies. We at Hölzel Diagnostika are your local sales partner and are happy to help you with any questions. Please do not hesitate to contact us.
At a glance:
| FANA oligos | siRNAs | |
| Transfection reagents | Not required | Indispensable |
| Specificity | Very high specificity for target DNA | siRNA class specificity |
| RISC-associated off-target effects | No | Yes |
| Transition from basic cell line to primary cells to animal models | One-step process, simple and user-friendly | Extensive optimization and use of delivery reagents required |
| Base cell lines | User-friendly handling – simply mix the FANA oligos with the cells | Transfection reagents required, optimization of the transfection reagents required |
| Primary cells and cells that are difficult to transfect | User-friendly handling – simply mix the FANA oligos with the cells | Transfection reagents are needed that can kill sensitive cells and change biology |
| Fish models | Works perfectly in fish models (especially zebra fish) | Not recommended |
| toxicity | Not toxic | Can be toxic |
| stability | Resistant to degradation by serum and cell nucleases | Moderate |
| Resources and time | Very easy optimization | Additional reagents and machinery needed, time consuming optimization |
| Cost savings | Cost effective application | Costly application |
Swell:
Eggert C. and Fischer U. (2003). RNA interference: a new tool for analyzing gene function. Biospectrum Heidelberg 9: 372-374.